Due to the presence of oppositely charged ions ionic compounds are held strongly by the electrostatic force of attraction. The peroxide anion is weakly bound to the cation and it is hydrolysed forming stronger covalent bonds.

Naming Ionic Binary Compounds Potassium Chloride Kcl Lithium Oxide Li 2 O Calcium Phosphide Ca 3 P 2 Silver Nitride Ag 3 N Copper Ii Iodide Cui 2 Iron Ppt Download
The bond formed between them is known as the ionic bond.
. The other oxygen compounds are also unstable in water. From the above example ionic compounds can be defined as the compounds formed by the transfer of electrons between metals and non-metals. Na 2 O 2 2H 2 O 2NaOH H 2 O 2.
Here both elements are ions an anion which has a negative charge and a cation which has a positive charge. 2KO 2 2H 2 O 2KOH H 2 O 2 O 2 Li 2 O H 2 O 2LiOH. The alkali metal peroxides are ionic compounds that are unstable in water.
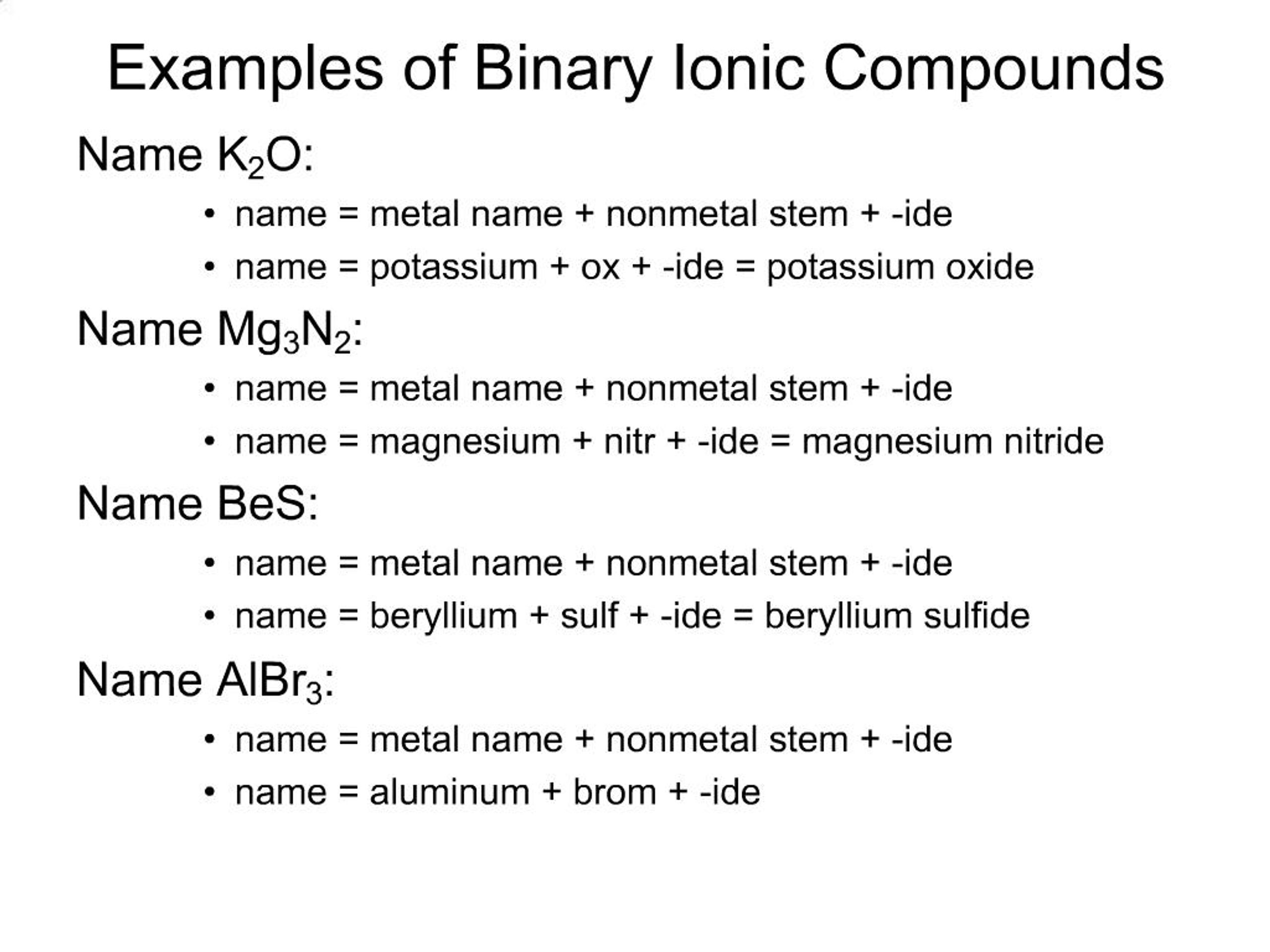
The binary compound list is mentioned in the table below. Binary ionic compounds are salts which consist of only 2 elements.

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Naming Ionic Compounds A Guided Inquiry Exercise

Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download
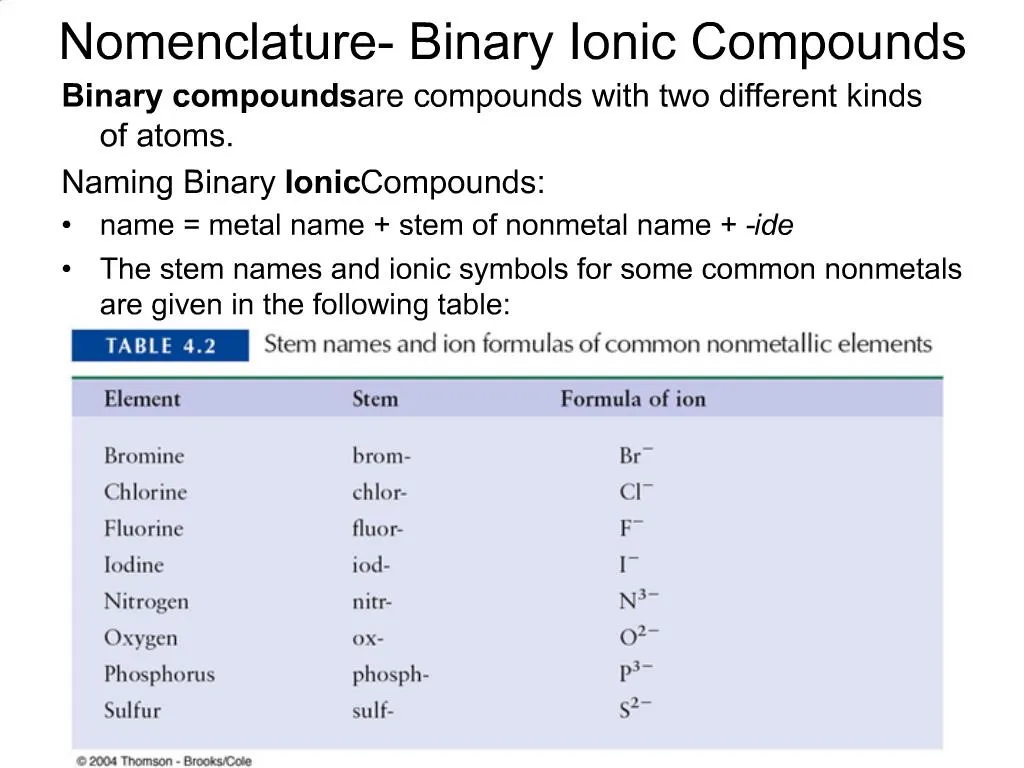
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132
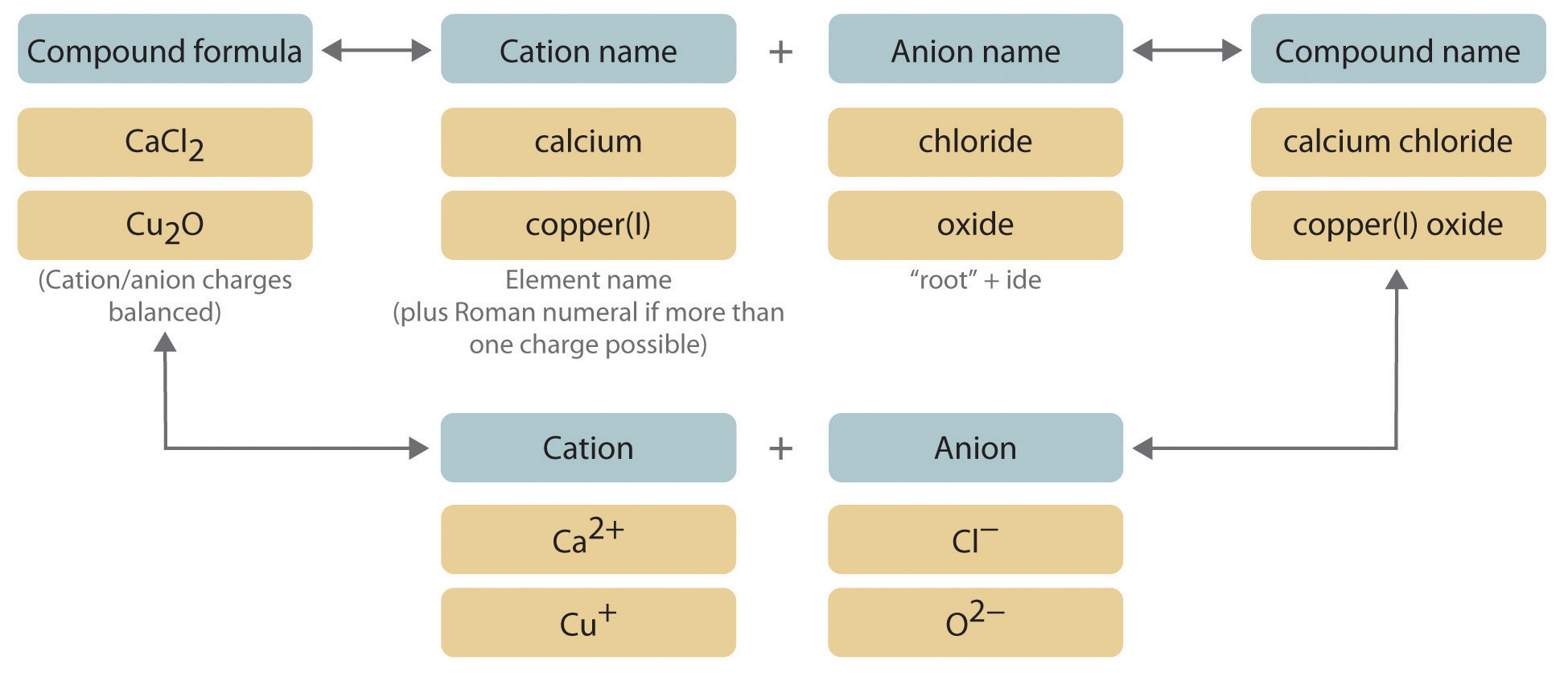


0 comments
Post a Comment